currently editing
Our postdoc Chiara Biagini and our former group member Francesca Della Penna, with the help of our PhD candidate Valeria Nori chairing the event, organised a seminar for students and PhD candidates on the transition between academia and job market. “Laurea, PhD: e poi?” (“Degree, PhD: what’s next?”) was held on 19 and 22 Feb 2021 and it was born from Chiara and Francesca’s experience in corporate recruiting, which they decided to share in order to help students cope with this delicate moment without panic.
Day one was dedicated to how to prepare at best for a job interview; day two was dedicated to the different role of chemists outside academia. On this second day, we could count on the contributions of three amazing guests: Dr Vittorio Maglia from Federchimica, Miriana Montefusco from Valagro‘s Talent Development Office, and Fernando Gomollon Bel, freelance science communicator and press coordinator for Graphene Flagship.
We had around 70 partecipants on both days and the live chat was burning with questions! It was such a success, congratulations to Chiara, Francesca and Valeria and thanks to our amazing guests!
Chiara’s passion for scientific dissemination meets her love for storytelling in this 2-minute spot.
A range of scientific curiosities and anecdotes are unraveled during “ChiaraMente: la scienza accessibile”, the weekly appointment curated by Chiara Biagini, our postdoc, on Radio UnivAQ.
If you speak Italian, or want to test your Italian understanding, you can enjoy it on Radio UnivAQ streamed here, every Thursday from 17:15 to 18:00.
Hedwig Lamarr – 21 May 2020
The protagonist of this first episode is Hedwig “Hedy” Lamarr, one of the most relevant examples in history of women’s multitasking abilities; worldwide famous actress and visionary inventress. After leaving her engineering studies in Vienna to pursue a career in cinema, Hedy becomes a Hollywood sex symbol, and is considered “the fairest woman of her times”. But, between a movie and another, her inexhaustible creative side and her scientific education led her away from the stages: from American patent offices to Howard Hughes’ planes. As a matter of fact, you may not know that the WiFi you might be using in this very moment is based on a wireless communication protocol which is rooted in one of her visionary inventions… Why not reading more on Wikipedia?
Chemiophoby – 28 May 2020
This second episode is dedicated to “chemiophoby”, the fear of chemistry, a common feeling which has grown widespread nowadays, and at the basis of which the struggle between “natural” and “artificial” lies. Is a substance synthesized in lab different from its natural homologue? And, most importantly, is the natural substance in principle better than the lab-made one? A two-minute journey through the story of Aspirin, the worldwide used anti-inflammatory drug, will try to explain why sometimes lab-made is better than natural. Even from the old ages, willow bark was known to contain a natural painkiller substance. But it was not until the mid-1800s, when someone found the way to synthesize it in the lab, that salicylic acid was available in large amounts suitable for the market. But chemistry didn’t give only a way to obtain this precious molecule without cutting any tree: thanks to some chemical modification on the molecule, “natural” salicylic acid became “artificial” acetylsalicylic acid, a substance which retained the positive effects of its precursor without the tremendous side effects on stomach that afflicted the users. So, what do you think – are those benefits enough to consider chemistry something not to be afraid of?
Baking Bread pt. 1 – 4 June 2020
Say goodbye to your badly leavened homemade loaves: in this short bread-themed miniseries, we will realize that baking bread is “simple” as chemistry! In this first episode we will focus on flour explaining how crucial it is to choose the right flour – parameters like protein content and W index need to be taken into account. And what happens during the first stages of mixing? Gliadin and glutenin (the proteins in the flour) absorb water, swell and get stretched during kneading, forming long fibers of gluten. A correct folding of the dough creates the right network of gluten which entraps the gas released by yeasts… By the way, yeast will be under the spotlight next week, so stay tuned!
Baking Bread pt. 2 – 11 June 2020
Second episode of the mini-series about the chemistry of baking! Have you ever asked yourself what yeast is, and how it works? Then this episode is right for you! Yeast is a living being composed of small micro-organisms which live on sugars and produce carbon dioxide gas, same as we do when breathing. Gluten helps entrapping carbon dioxide bubbles into the dough, making it leaven. Temperature is crucial in order to obtain the perfect rising… and be aware that if you add too much salt you may kill the yeast! Are you curious now about how to combine flour and yeast correctly? Don’t miss the next episode about kneading!
Baking Bread pt. 3 – 18 June 2020
Final episode of this mini-series about the science of bread! Every gesture in kneading has its own importance and meaning. Not only stretching the dough is necessary in order to form gluten, but also folding the dough after raising is essential in order to re-distribute gluten in layers and help the dough being stable during the raise in height. But what if your loaf didn’t rise at all? It could be because of the salt, which might have killed the yeast. Or else, if you added milk to make your dough fluffier, you might accidentally have warmed it a bit too much, which could be the reason why the yeast didn’t work. And if you added oil or butter to your dough, you might have added it in the wrong order. In fact, every fatty substance should be added to the dough only once the gluten has been formed, otherwise it would entrap starch granules in an oily membrane making them unable to interact with water and form gluten. Now you’re ready – go and bake your perfect loaf!
The secret life of sunflowers – 25 June 2020
Do you know why sunflowers follow the sun? Or, at least, the young buds do – the adult flowers don’t… This behavior is known as “Heliotropism” (from Greek, “following the sun”) and is common in many flowers and plants as it brings many benefits: warm petals are attractive for pollinator insects, sun is thought to enhance pollen germination, and opposing the corolla to the sun with a certain angle casts a shadow on the most delicate part of the flower. But sunflower has another reason for doing this: it is known that young sunflowers’ growth is regulated by an internal clock with cycles of approximately 24 hours. During this cycles the growth of the stem is curiously uneven: one side grows during the day, the other during the night, hence the oscillation of the bud. Once the growth is complete, the stem becomes less flexible and the corolla fixes definitely its orientation towards east, where the rising sun warms the flower in order to make it more attractive to morning-active insects. Could you imagine that a flower’s life could be so active?
A journey into colour pt. 1 – 2 July 2020
We usually say “a red flower”, “blue sky” – but did you know that colour is, to be precise, a feature of light? Light is composed of extremely small particles of a certain energy, which is referred to as “frequency”, and, basing on this, all light can be divided into several categories – actually “visible light”, the one associated to colours, is only a small fraction of the existing kinds of light. We can say that colour is the way we can perceive different frequencies of light. So, how can we describe an object as coloured? Most if the time, in nature, colour is due to the presence of pigments, molecules which are able to absorb light but to reflect or re-emit only a fraction of it. Due to the absorbing action of pigments, the colour we see is actually what remains from the incident, white light. Sometimes, though, Nature plays jokes to our eyes and makes us see colours which do not exist from a chemical point of view. To find out how, wait for the next episode!
A journey into Colour pt. 2 – 9 July 2020
In 1665, the English scientist Robert Hooke was investigating the stunning colours of a peacock feather, finding out two interesting things: first, while the feather appeared smooth on the surface, it was composed of an array of layered microstructures similar to granules if observed under the lens of the microscope; second, the blue-green iridescence disappeared if the feather was put under water. The reason for this behavior was ascribed to light and it became a matter of great interest and debate through years. Now we know that, while the feather is normally brown due to the presence of melanin as a pigment, light behaves strangely when interacting with the regular micro-array of granules, creating those strange optical effects of colour-without-pigment. Structural colour, as it is called, is widely used among birds, beetles, butterflies, flowers and berries, to create striking colour effects. Furthermore, it lies at the basis of the amazing camouflage effects of several animals. Man cannot control colour change such as chameleons do… but has learnt how to change the colour of objects in several ways. Find out more in the next episode!
A journey into Colour pt. 3 – 16 July 2020
Man is not able to change his colour in the same way animals do, but he discovered his own way to do it – by changing clothes! Colourants are one of the most ancient inventions of man, but bright, saturated colours were normally very expensive and therefore reserved for rich people. Synthetic colourants made coloured clothes affordable for everyone and represented a huge turning point in human history: it all began with mauveine, a molecule accidentally discovered by an 18-year-old English boy named William Perkin while doing his chemistry homework. After this colourant was released on the market, causing the spread of the so-called “Mauve measles”, many other synthetic dyes followed afterwards, giving people an unprecedented right – the right to be colourful!
What’s in your solar filter? – 23 July 2020
In 1938, the Austrian chemist Franz Greiter got a massive sunburnt from his trekking on the top of a glacier in the middle of the Alps. This episode gave him the idea to develop, years later, the first modern solar filter named after the glacier itself – Piz Buin. Many steps forward have led us to our modern solar filters: first of all, the assessment of the so-called SPF factor, the number which identifies the percentage of ultra-violet rays blocked from the filter. Did you know that SPF 30 means that only 1/30 of the UV-B radiation passes through the filter and arrives on our skin? And that the appropriate amount of cream to be applied is crucial, otherwise the filter would lose its efficiency? You should apply each time around 30 grams – approximately a ping-pong ball. Last but not least: have you ever wondered which are the molecules in your filter which are actually responsible for the protection? They can be both small insoluble particles which are able to reflect light before it is absorbed by skin, or bigger molecules which act as antennae for UV rays – they absorb them and disperse them as innocuous heat. Whatever cream you choose, make sure you use it correctly!
Ice cream… as you’ve never seen before – 30 July 2020
Warning: your ice cream contains a lot of chemistry! Even if defining the typical “ice cream” is quite a complex matter, because of the huge variety available, scientists agree on one point: it is “one of the most complex foods ever”. Ice cream contains all the three states of matter combined in many different ways: solid ice crystals, syrups, eggs and milk for the liquid part and… air, lots of air, which gives this dessert his luscious softness. Such a complex mixture is not easy to handle, as complex physico-chemical studies have to be performed in advance in order to provide the user the best tasting experience. So, next time you eat your favourite ice cream, remember to thank scientists!
The Pint of Science festival aims to deliver interesting and relevant talks on the latest science research in an accessible format to the public – mainly across bars, pubs, cafes and other public spaces. Pint of Science wants to provide a platform which allows people to discuss research with the people who carry it out and no prior knowledge of the subject is required. It is a network of thousands of volunteers who are passionate about bringing discoveries to people and was established by a community of postgraduate and postdoctoral researchers in 2012.
Armando was the Pint of Science coordinator in Cambridge (UK) from 2014 to 2016; the following year, when he moved back to Italy, he contributed to start Pint of Science in L’Aquila as well.
Creative Reactions is the science-meets-arts branch of Pint of Science, encouraging collaboration between artists and scientists to showcase research in a more creative manner. Armando conceived and started it in Cambridge during Pint of Science 2015 when speakers at the festival were paired with local artists to showcase their research, and proved a major success. Nowadays, Creative Reactions has spread to a number of cities. Each event has a different format – expect anything from a traditional Pint of Science-style evening, to dramatic performances, music, comedy and even full art exhibitions with the artwork for sale.
What happens when you mix chemistry and cocktails? Armando conceived and developed special events during Pint of Science 2016 in Cambridge, in collaboration with Compound Interest. During the nights, they unveiled the chemical secrets behind what makes molecular cocktails taste and look so good, while professional mixologists demonstrated techniques such as gelification and spherification, or even how to make tasty foams! You can find more info and the infographics from Compound Interest here
Taking the experience from Science Hits the Bar to L’Aquila, Armando organised few events, in 2018 and 2019, and during Street Science 2019, to discuss the magic that chemistry can bring in a cocktail – different techniques and cocktails were discussed and explained at each event, guided by the mixologists from Il Vermuttino.
Fame Lab is an international science communication competition – Chiara Biagini took part in the selections in L’Aquila in March 2020, talking about the “vegetarian dilemma” and how the dyes came to be used daily in our society
When COVID-19 is giving very hard times to Italy and the entire world, Chiara Biagini, in collaboration with Compound Interest, prepared the Italian version of the infographic “Coronavirus: How hand sanitisers protect against infections”. Using Compound Interest’s words: “As coronavirus continues its spread, panic-buying has swept supermarket shelves of hand sanitisers. What’s in these sanitisers and how effective are they in comparison to hand washing? This graphic takes a look.”
Chiara also contributed to the Italian version of “How do tests for coronavirus work?” – Once again, as Compound Interest put it “At the time of writing, there have been almost a quarter of a million confirmed cases of COVID-19 worldwide. The number of deaths is approaching 10,000. Across the world, countries are scrambling to increase their testing capacity for the virus — how are these tests carried out and how do they work?”
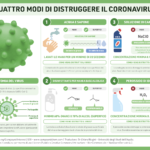
Another infographic that Chiara translated into Italian is “Four ways to destroy coronavirus” –“How do you fight something you can’t see? That’s the question when it comes to the coronavirus crisis which currently has many of us holed up at home.”. This time Compound Interest helps us to understand four ways to destroy the coronavirus from our hands and from surfaces, along with the science behind it.